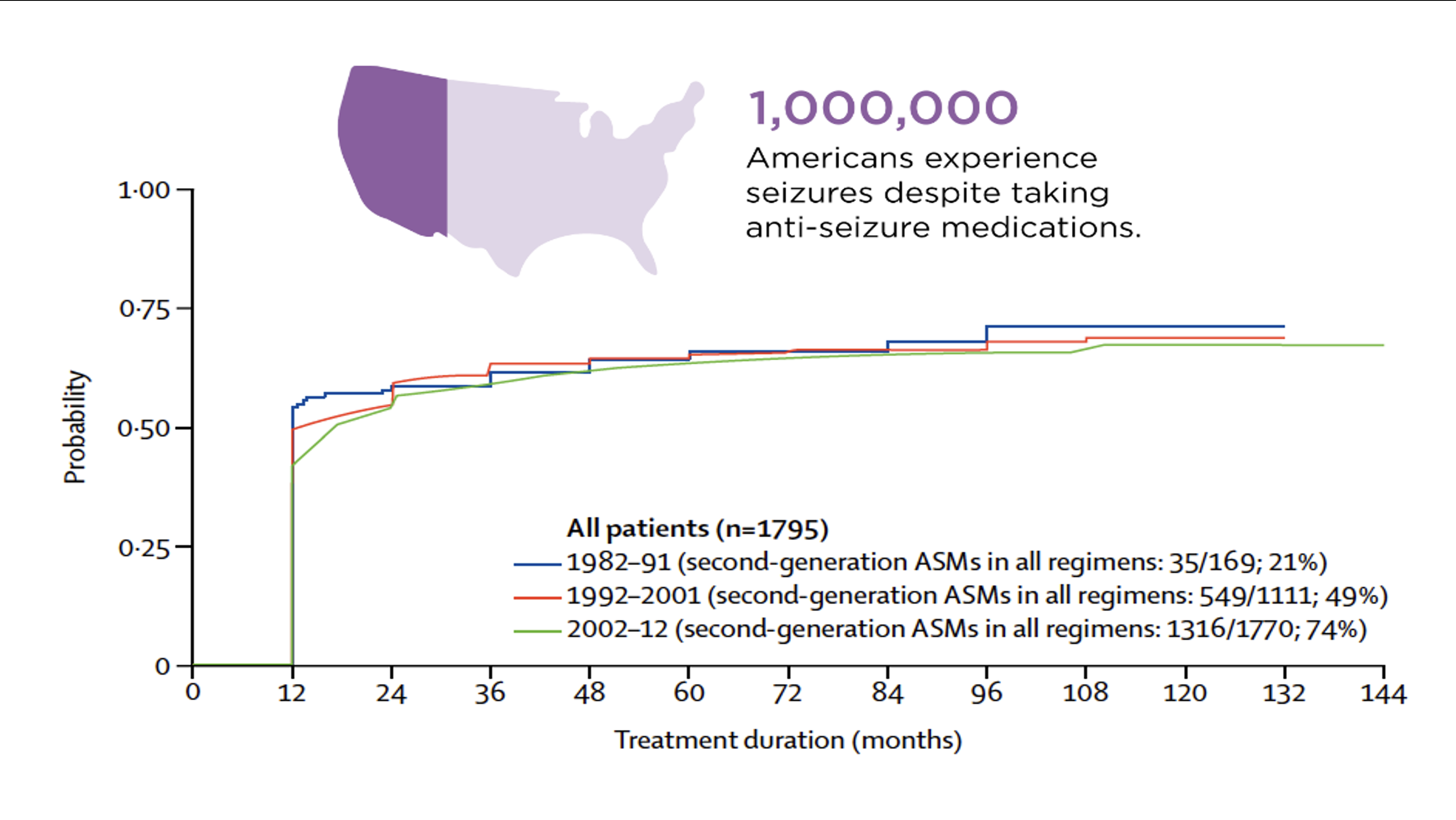
Epilepsies are neurological conditions associated with hypersynchronous neuronal activity that can propagate throughout the brain, interrupt consciousness, and produce motor convulsions in some patients. These seizures and other symptoms associated with epilepsy can be severely limiting, and thus constitute an enormous social and financial burden on families and society. Epilepsy has traditionally been treated with anticonvulsants, many of which target the same inhibitory pathways. Despite dozens of existing FDA-approved anticonvulsants, these medications fail to appropriately manage epilepsy in about one third of adults and 25% of children, leading to what is known as drug-resistant or refractory epilepsy. Furthermore, the fraction of patients with seizures that are refractory to medication has remained stubbornly constant at ~30% over the past 30 years, despite the introduction of new medications (Figure 1). In the United States, over 1 million patients have inadequate seizure control.
Historical and recent clinical evidence supports the use of cannabidiol (CBD), a component of Cannabis, as an anticonvulsant agent. A substantial amount of data in the academic literature confirms that CBD has beneficial actions at concentrations that show low abuse potential. Moreover, CBD has molecular targets that are distinct from those of existing anticonvulsants. However, CBD is a highly lipophilic molecule with physicochemical properties that severely compromise its pharmacokinetics. It is poorly absorbed, rapidly metabolized, resulting in unusually low bioavailability. Even with these existing problems, GW Pharma brought CBD to market as formulated as Epidiolex® (now Jazz Pharmaceuticals) and it has been shown to still effective in ~35% of patients with three rare genetic, medically refractory epilepsies – Dravet and Lennox-Gastaut Syndrome, and Tuberous Sclerosis Complex.
Historical and recent clinical evidence supports the use of cannabidiol (CBD), a component of Cannabis, as an anticonvulsant agent. A substantial amount of data in the academic literature confirms that CBD has beneficial actions at concentrations that show low abuse potential. Moreover, CBD has molecular targets that are distinct from those of existing anticonvulsants. However, CBD is a highly lipophilic molecule with physicochemical properties that severely compromise its pharmacokinetics. It is poorly absorbed, rapidly metabolized, resulting in unusually low bioavailability. Even with these existing problems, GW Pharma brought CBD to market as formulated as Epidiolex® (now Jazz Pharmaceuticals) and it has been shown to still effective in ~35% of patients with three rare genetic, medically refractory epilepsies – Dravet and Lennox-Gastaut Syndrome, and Tuberous Sclerosis Complex.
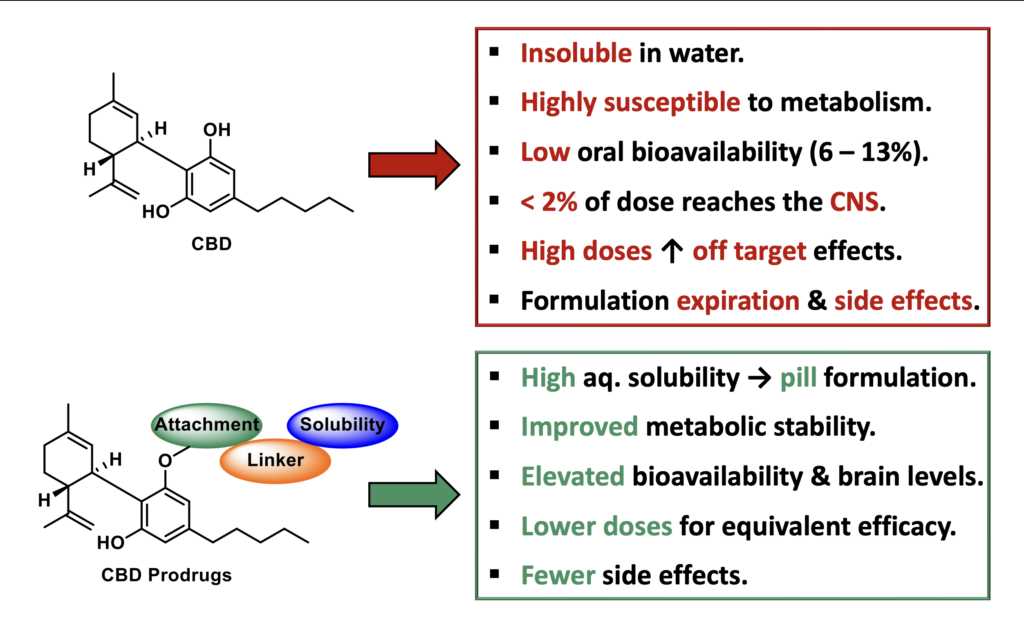
While effective, CBD is highly lipophilic with poor physicochemical properties that compromise its pharmacokinetics (Figure 2). CBD is virtually insoluble in aqueous media (17µM), which requires Epidiolex® to be formulated and administered in a sesame oil-based liquid that expires after 12 weeks. CBD is poorly absorbed, accumulates in the liver with significant first pass metabolism, shows a high liver/brain ratio, and is associated with gastrointestinal side effects. All of this results in a very low oral bioavailability (6 – 13%), limiting its clinical efficacy. Consequently, high doses of CBD are required to elicit an effect in patients, increasing the possibility of off-target side effects and drug-drug interactions. This is exacerbated by the fact that CBD acts an inhibitor of CYP3A4 which metabolizes numerous CNS acting drugs and co-administration with other anticonvulsants can lead to the development of adverse side effects. These poor drug-like properties result in sub-optimal treatment and lost revenue and patients. Collectively, there is a great need for improved CBD-based therapeutics with enhanced physicochemical and pharmacological properties, for treating epilepsies, especially medically refractory epilepsy.
In the Liotta Research Group, we propose to apply our substantial experience with prodrug synthetic strategies to develop a novel CBD prodrug that is water soluble, allows for conventional formulation, improves oral bioavailability, and modifies tissue distribution in a favorable manner (Figure 2). This prodrug will be able to more predictably and effectively treat intractable epilepsies that are currently treated with Epidiolex®, as well as treat developmental epileptic encephalopathy (DEE), which qualifies for orphan drug status. Our approach makes use of dual-functional moieties with controlled cleavage mechanisms placed onto the phenolic hydroxyls of CBD, to create structurally novel CBD prodrugs which are highly soluble and orally bioavailable. This is achieved through careful selection and design of a solubilizing, circulating linker and attachment group (Figure 3).
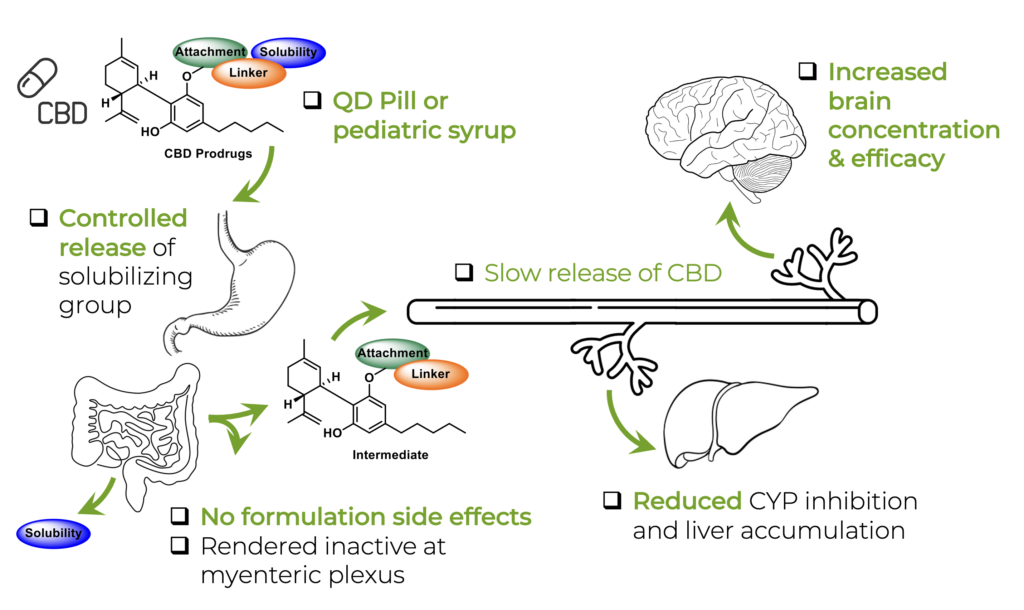
Throughout the development of this prodrug technology, our lead class has been identified as phosphate-containing prodrugs (Figure 4A). Over 60 prodrugs bearing numerous functional connecting groups, linkers and the terminal phosphate solubilizing groups have been synthesized, screened, and progressed to in vivo efficacy studies. The success of this specific prodrug class lies in its design principle, which facilitates dramatically enhanced solubility and allows for control of the release kinetics of both the phosphate solubilizing group (in the intestine) and the intermediate linker group following prolonged circulation and enhanced brain penetration (Figure 3). This is achieved by either the incorporation or exclusion of sterically demanding groups adjacent to CBD or the phosphate group, and the exchange of the connecting groups (esters, carbonates, and carbamates). This allows us to control release of the dual component prodrug differentially, which we are using to rationally design prodrugs with different temporal profiles for CBD release.
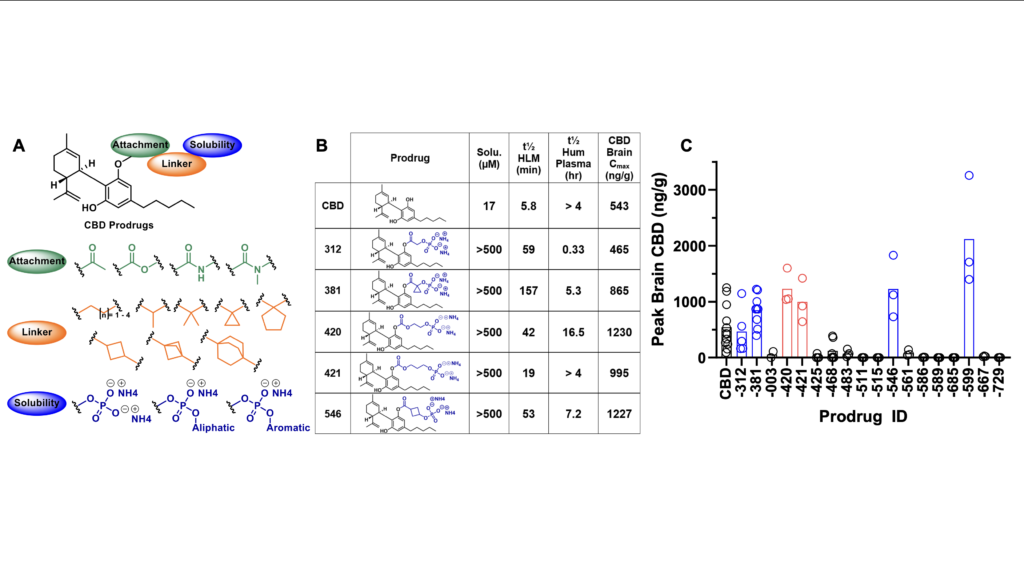
The effectiveness of phosphate-based CBD prodrugs is exemplified in Figure 4B, where chemical groups added to CBD from our lead class of prodrugs improve solubility and liver microsomal stability, are orally available, and produce a range of CBD levels in brain, which for 5 of 6 prodrugs is higher than those found with administration of CBD alone at a 50 mg/kg equivalent oral dose (Figure 4C).The phosphate, which is charged at physiological pH (but not fully at stomach pH) greatly improves solubility but does not hinder oral availability, as it is removed prior to absorption by intestinal phosphatases. While CBD inhibits CYP3A4, which metabolizes anticonvulsants such as carbamazepine and clobazam, our phosphate CBD prodrugs did not inhibit a panel of eight CYP enzymes (data not shown). We also did not observe inhibition by prodrugs of transporters OCT1, OCT2, OAT1, OAT3, BCRP, MATE1, MATE2-K; while both CBD and several prodrugs inhibited OAT1PaB1, OAT1p1B3, and BSEP (not shown). CBD prodrugs are not P-gp inhibitors nor efflux substrates, with LEH-381 showing a 3-fold improved efflux ratio over CBD (data not shown).
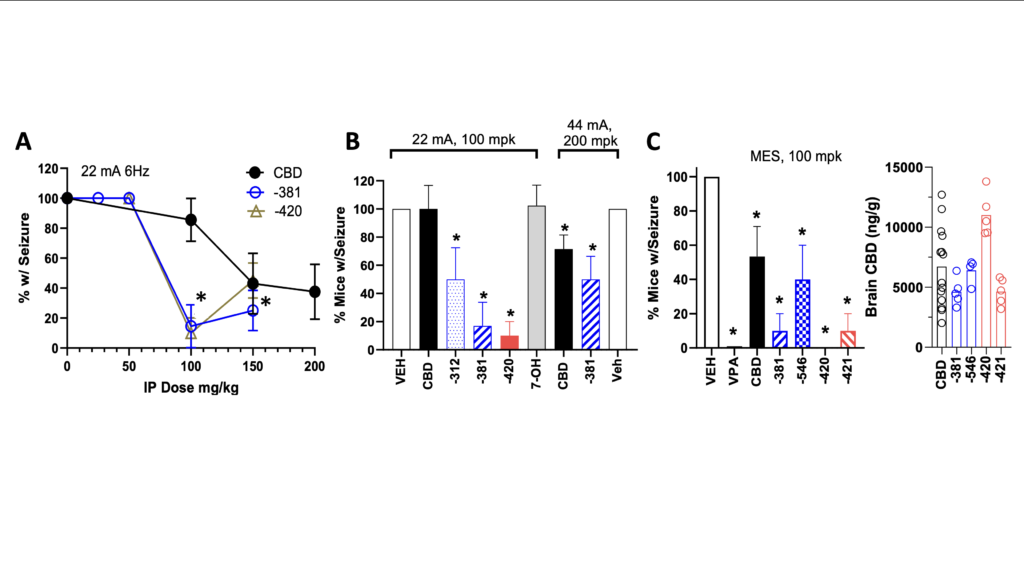
We have utilized the same 3 seizure models for evaluation of anticonvulsant actions of CBD prodrugs (6Hz 22mA electroshock, 6Hz 44mA electroshock and maximal electroshock or MES) that led to the clinical trials of Epidiolex®. These models utilized IP administration of CBD 2 hours prior to seizure. We observed anticonvulsant properties for all CBD prodrugs tested for protection utilizing Racine score for 6 Hz models and tonic hind-limb extension for MES, two of which were more potent at attenuating seizures than CBD alone, with statistically significant differences apparent at 100 mg/kg doses (Figure 5). The increased potency of LEH-381 raises the possibility that the intermediate following removal of the phosphate and prior to conversion to CBD may have anticonvulsant activity, adding a new dimension to prodrug efficacy. We are currently in the process of evaluating this hypothesis. Post-MES seizure monitoring of brain CBD confirmed prodrugs drove similar or higher CBD levels than CBD alone (Figure 5C, right panel). This data confirms that our strategy yields orally available agents that produce substantial CBD levels in brain, are more potent anticonvulsants than CBD, have reduced potential for CYP and transporter inhibition, and diminish CBD accumulation in liver.
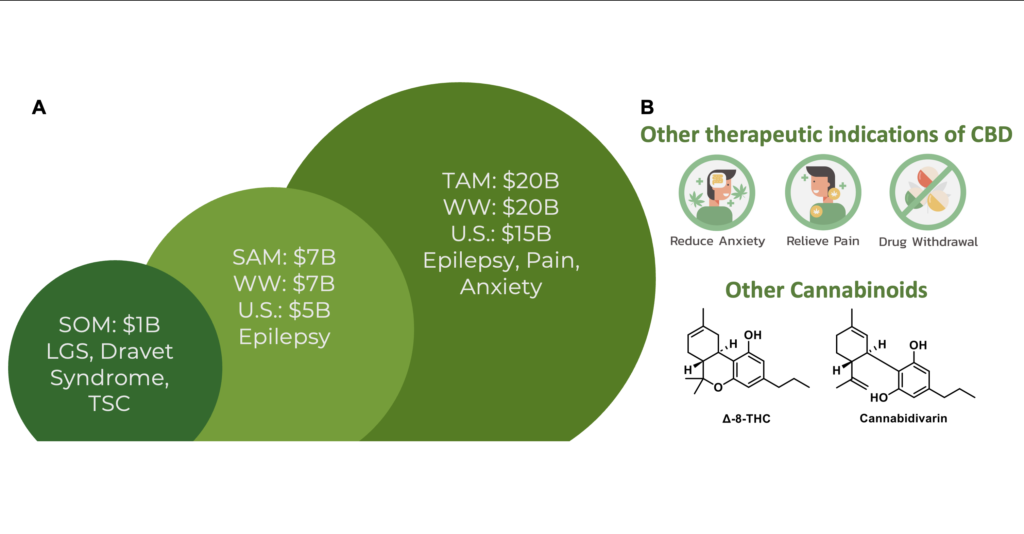
There are 3.4 million patients with epilepsy in the US, representing a total of 1.2% of the US population. Despite dozens of FDA-approved anticonvulsants, the fraction of patients that are refractory to treatment has remained constant at ~30% over the past 30 years. This problem reflects that many newly approved drugs share the same mechanism as existing therapies. CBD acts on molecular targets distinct to those of anticonvulsants and thus, development of novel CBD prodrugs, could be enormously beneficial for patients with refractory epilepsy. We estimate the market opportunity to treat DEE (refractory epilepsy subtypes) at approximately $1B WW, with expansion to other epilepsies as an opportunity approaching $7B WW (Figure 6a). The CBD-prodrug may have further clinical applications to pain and anxiety which in aggregate constitutes a $20B WW market. Lastly, CBD prodrugs act as a true platform technology in two ways, the first being the clinical application of CBD prodrugs for other indications such as pain and anxiety, accessing multiple markets from one candidate (Figure 6B).